Lupkynis (voclosporin): This immunosuppressant medication, approved in 2021, features a microemulsion formulation that improves oral bioavailability. It's used for preventing rejection in kidney transplant recipients. Its applicant holder is Aurinia Pharmaceuticals. Inc, and application number is N213716. It has one strength, and it is 7.9 mg.
Orange Book Patents
Lupkynis is covered by 3 orange book patents - 7332472 (exp. in 2024), 10286036 (exp. in 2037) and 11622991 (exp. in 2037). 7332472 claims "A composition comprising an isomeric mixture of a cyclosporine analogue modified at the 1-amino acid residue with a 1,3-diene substituent, wherein the isomeric mixture comprises about 90% to about 95% of the E-isomer and about 10% to about 5% of the Z-isomer, wherein the isomers are the isomers E- and Z", see below:"
Sales
Aurinia issued a press release that Aurinia increased its net product revenue guidance to a range of $150 - $160 million for net product sales of LUPKYNIS. The guidance range was based on assumptions regarding PSF run rates, consistent conversion rates, time to convert, persistency, and pricing. (1)
LUPKNIS - API and Product Ingredients
LUPKYNIS (voclosporin) capsules is available for administration as soft gelatin capsules containing 7.9 mg voclosporin per capsule. Inactive ingredients include alcohol, Vitamin E polyethylene glycol succinate (NF), polysorbate 40 (NF), medium-chain triglycerides (NF), gelatin, sorbitol, glycerin, iron oxide yellow, iron oxide red, titanium dioxide, and water.
Voclosporin appears as white to off-white solid matter. At ambient temperature, voclosporin is freely soluble in acetone, acetonitrile, ethanol, and methanol, and practically insoluble in heptanes (USP). Voclosporin is practically insoluble (less than 0.1 g/L at 20ºC) in water and melts above 144ºC with decomposition.
LUPKNIS - PK data
- With a twice daily dosing regimen, voclosporin achieves steady-state after 6 days and the accumulation is approximately 2-fold.
- The median T max of voclosporin is 1.5 hours when administered on an empty stomach.
- Co-administration of voclosporin with food decreased both the rate and extent of absorption: with meals, C max and AUC of voclosporin were reduced by 29% to 53% and 15% to 25%, respectively.
- Protein binding of voclosporin is 97%. Voclosporin partitions extensively into red blood cells, and half-life is about 30 hours.
- Voclosporin is predominantly metabolized by CYP3A4. CYP3A4 is a crucial enzyme in drug metabolism and detoxification, primarily resides in two key locations - liver and small intestine.
- Following single oral administration of radiolabeled voclosporin 70 mg, 92.7% of the radioactivity was recovered in feces.
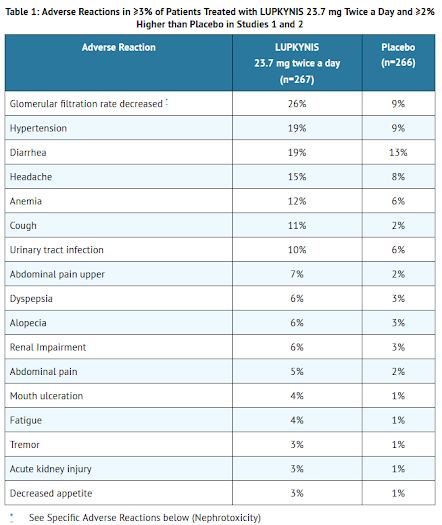
Adverse Reactions
For details, please check with the product insert of LUPKNIS.
Reference
(1) Aurinia Pharmaceuticals Reports Second Quarter and Six Months 2023 Financial and Operational Results, Biospace.com, Aug 03, 2023.