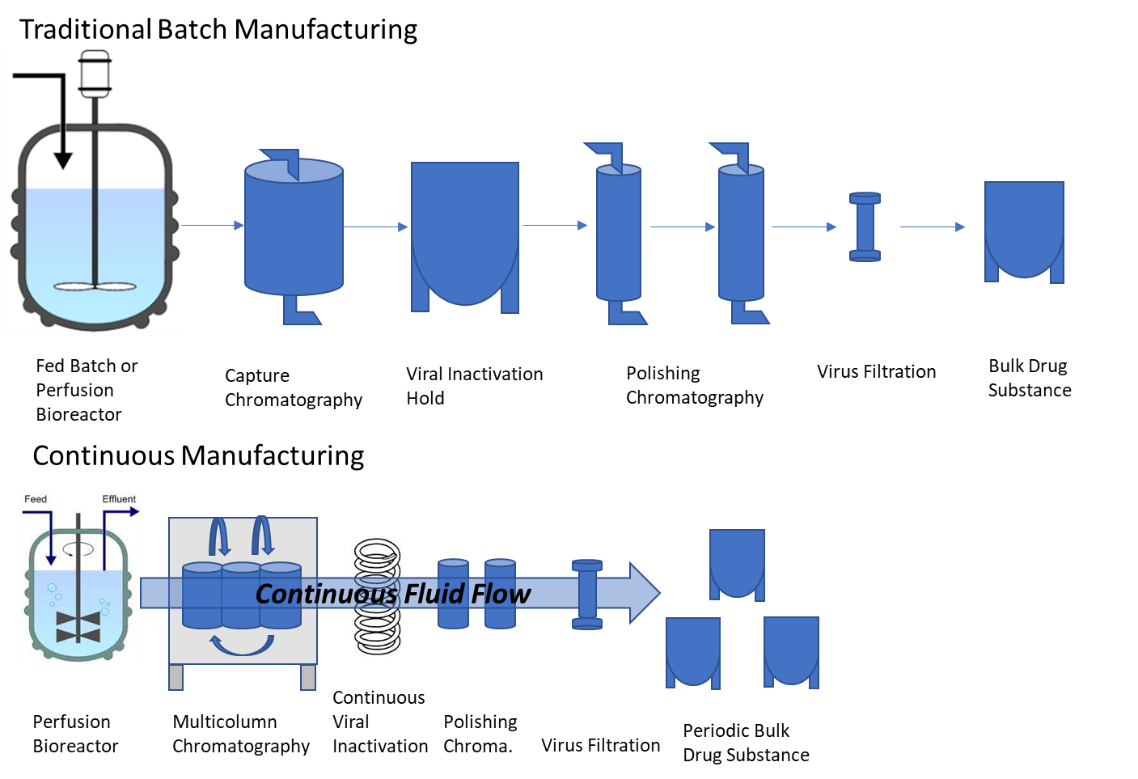
Shifting Therapeutic Protein Drug Substance Manufacturing Paradigms
While traditional therapeutic protein manufacturing involves producing the protein in engineered cells followed by purification to remove product- and process-related impurities, including potentially infectious viral particles (such as retrovirus-like particles, RVLPs). Guidance document ICH Q5A takes a three-pronged approach to address viral safety risks: i) starting material testing (i.e., cell bank testing), ii) raw materials and intermediates testing, and iii) viral clearance testing during the purification process. The purification process includes both non-dedicated (i.e., chromatography) and dedicated virus clearance steps (i.e., chemical inactivation and virus filtration) to effectively clear RVLPs (and any possible viral contaminants) from the final drug product.
The emerging continuous manufacturing approach necessitates new strategies. This integration of production and purification allows for shorter processing times, reduced manual intervention, and a reduction in facility size through the use of smaller tanks, bioreactors, and columns. However, continuous manufacturing continuously purifies the protein during production, posing unique challenges for ensuring viral safety. Regulatory frameworks and testing methods developed for traditional methods may not directly translate to continuous manufacturing, hence the need for ongoing research and collaboration between manufacturers and regulators to establish robust viral safety measures for this innovative approach.
CDER Researchers' Study
Continuous manufacturing (CM) presents unique challenges for ensuring viral safety due to its dynamic nature compared to traditional batch processing. To address this, CDER researchers identified three key steps in CM: capture chromatography, viral inactivation, and viral filtration. They adapted existing batch-based methods to these continuous steps, developing small-scale models for studying their effectiveness.
One focus area was continuous capture chromatography, which uses multiple columns cycled through various stages. Initial concerns existed about its impact on viral safety. However, side-by-side comparisons with batch processing showed similar viral clearance capabilities, suggesting minimal impact and the feasibility of using simulated batch-based models for evaluation.
This research highlights the ongoing efforts to ensure viral safety in continuous manufacturing and the development of appropriate testing methods for this innovative manufacturing approach.
Similar to capture chromatography, continuous viral filtration in continuous manufacturing raises concerns due to its unique operating features. These include longer processing times, fluctuating product streams, and continuous flow instead of pressure. Researchers addressed this by developing two small-scale models mimicking specific aspects of continuous filtration.
One model simulated longer processing times and volume throughput. Over four days, it maintained pressure within limits and effectively cleared the viral surrogate, demonstrating the feasibility of long-term continuous filtration.
The other model focused on dynamic product streams, replicating fluctuations in protein and virus concentration. While pressure was affected, viral clearance remained robust, suggesting minimal impact on safety.
These protein-specific models provide a starting point for understanding continuous filtration and can be adapted to different systems, ensuring viral safety as continuous manufacturing technology advances.
Conclusion
As continuous manufacturing gains traction for therapeutic proteins, limited research exists on ensuring their viral safety. This study provides valuable insights for both internal and external stakeholders, shedding light on potential concerns and methods for safe development using continuous manufacturing. With increasing submissions incorporating continuous manufacturing elements, readily available FDA expertise and public examples become crucial for successful implementation and regulatory evaluation. This research aligns with the Emerging Technology Program's goals, paving the way for safe and efficient continuous manufacturing adoption.
Figure 1. Comparison of batch manufacturing to continuous manufacturing. (Credit: FDA)